38 fda health claims on food labels
Fda Health Claims On Food Labels FDA Proposes to Update Definition for "Healthy" Claim on … Health (7 days ago) September 28, 2022. The U.S. Food and Drug Administration today issued a proposed rule to update the definition of the nutrient content claim "healthy.". Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are...
Fda health claims on food labels
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food... Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the...
Fda health claims on food labels. FDA perspectives on health claims for food labels - PubMed The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims charact … Enforcement Policy Statement on Food Advertising The Federal Trade Commission (FTC) is issuing this statement to provide guidance regarding its enforcement policy with respect to the use of nutrient content and health claims in food advertising. The Commission believes the statement is appropriate in light of the passage of the Nutrition Labeling and Education Act of 1990 (NLEA), 1 and the ... 5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." Use of the Term Healthy on Food Labeling | FDA The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance. Updating the "healthy" claim...
Fda Health Claims Food The FTC regulates all forms of advertising. FDA regulates the content and form of labels for food, cosmetics, dietary supplements, devices and drugs.Labels are simply a special form of advertising, so both FTC and FDA regulate them.FDA reviews labels for compliance and to see that you meet the basic requirements to be in the market. Health Claim On Food Labels Health Claims On Food Labels PubMed. Health Health claims on food labels Mil Med. 1994 Mar;159(3):213-7. Author L Tollefson 1 Affiliation 1 Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC … Detail: Visit URL . Category: Health View Health What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims) Definitions - Health claims on food labels - Food labels - Canadian ... Claims that refer to the specific beneficial effects that the consumption of a food or food constituent has on normal functions or biological activities of the body. Such claims relate to a positive contribution to health or performance. For example, " [Naming the food or food constituent] promotes regularity or laxation".
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Health Claims on Food Labels | LegalMatch In short, yes. A health claim must be approved by the Food and Drug Administration ("FDA") before the manufacturer is allowed to put the health claim on one of their food products. In general, there are two ways in which a manufacturer can obtain FDA approval: Scientific Data and Evidence: The most common way in which a manufacturer can ... Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... FDA Proposes New 'Healthy' Claim on Food Labels Regulatory Compliance FDA Proposes New 'Healthy' Claim on Food Labels Sept. 28, 2022 Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging.
FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages to ... Under the proposed definition for the updated "healthy" claim, which is based on current nutrition science, more foods that are part of a healthy dietary pattern and recommended by the Dietary...
FDA Proposes to Update Definition for "Healthy" Claim on Food Labels FDA Proposes to Update Definition for "Healthy" Claim on Food Labels Constituent Update September 28, 2022 The U.S. Food and Drug Administration today issued a proposed rule to update...
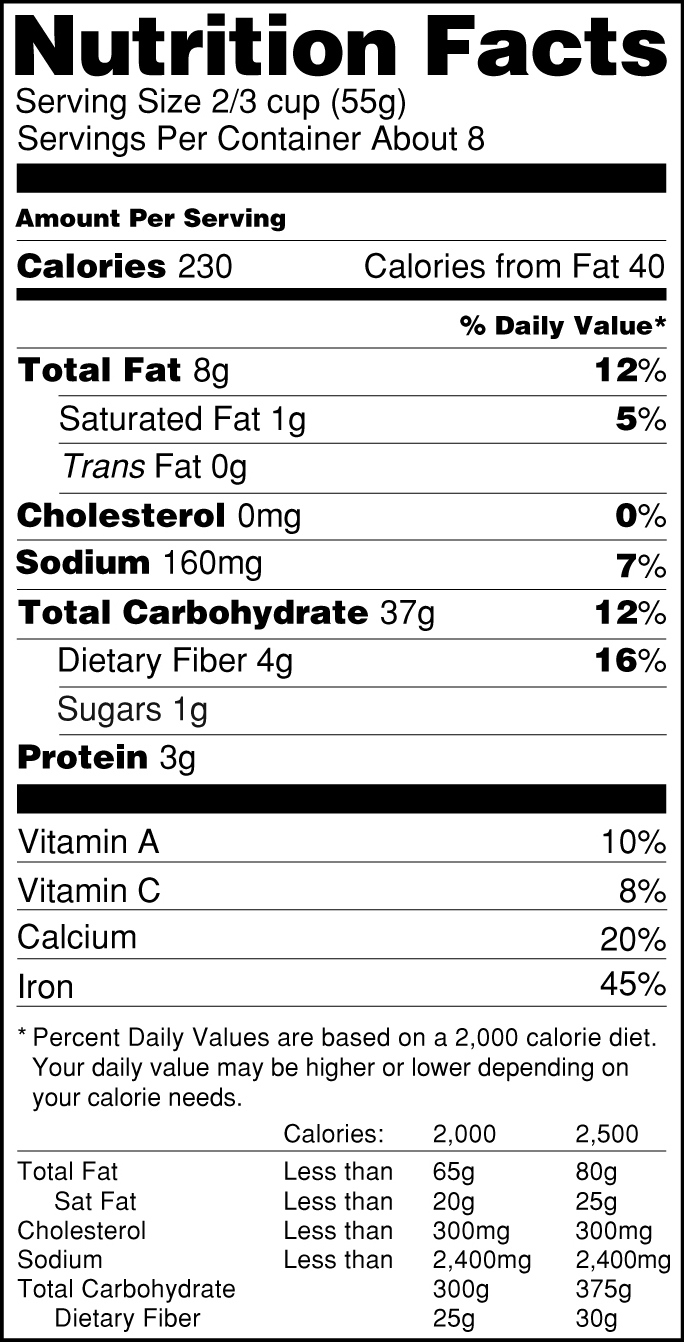
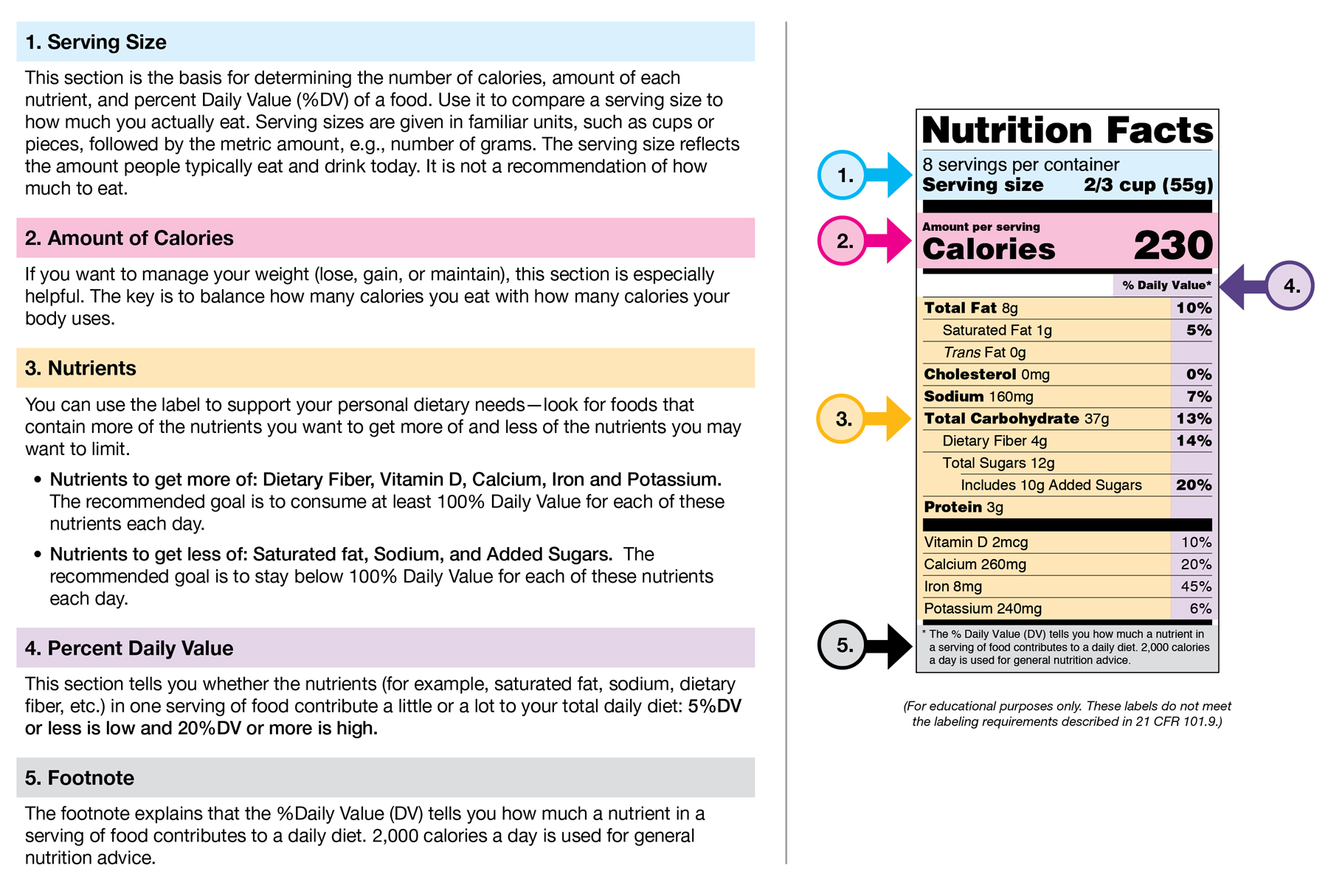
Everything you need to know about Health Claims on Food Labels A nutrient content claim must be true and accurate just like health claims, it is a statement about the amount of a nutrient found in a food. Usually placed on the front of the food label, the nutrient claim provides a quick comparison between similar products. Some examples of nutrient claims are "low sodium", "high in fiber", " fewer calories",
FDA proposes updates to 'healthy' claim on food packages | CNN In order to be labeled with the "healthy" claim, products would need to: Contain a certain, meaningful amount of food from at least one of the food groups or subgroups - such as fruits,...
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
FDA Permits Qualified Health Claims on Nut Labels For the first time, the U.S. Food and Drug Administration has recognized a qualified health claim made by a conventional food. In a surprise move in July the FDA granted permission for peanuts, almonds, hazelnuts, pecans, pistachios and walnuts to carry a label touting their heart-healthy effects. "Scientific evidence suggests but does not ...
Fda Health Claims For Food Questions and Answers on Health Claims in Food … Health (8 days ago) 4. Has the FDA ever revoked an authorized health claim? The FDA has authorized 12 health claims since 1990. On October 31, 2017, the agency issued a proposed rule to revoke the regulation that
Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written...
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the...
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food...
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...































Post a Comment for "38 fda health claims on food labels"